
C Heather Ashton DM, FRCP, Department of Clinical Psychopharmacology, University of Newcastle, England
First Published in New Scientist, May 6, 1989
Valium, Librium and Mogadon once seemed to provide the perfect answer to stress. We now know how this group of drugs alters the chemistry of the brain; no wonder they create more problems than they solve
The 1950s witnessed the explosive discovery of a series of new drugs that changed the face of psychological medicine. Among the eruptions were the antidepressant drugs, now used extensively to treat depressive illnesses; the antipsychotic drugs, also known as major tranquillisers, which transformed treatment of schizophrenia; and the so-called minor tranquillisers, apparently the panacea for all forms of anxiety. One of the first minor tranquillisers was chlordiazepoxide (tradename Librium), released in Britain in 1960. A host of related drugs followed, all with the same basic benzodiazepine structure. and the term tranquillisers is now synonymous with the generic name benzodiazepines.
When they first appeared, the benzodiazepines, more than most drugs, appeared to be “wonder drugs”. They calmed the mind, relaxed the muscles, controlled insomnia, helped people to tolerate the stresses of life, and were also useful anticonvulsants for people suffering from epilepsy. The drugs were remarkably safe—a handful of tablets taken in overdose usually produced little more than a long sleep—and their potential to produce dependency appeared to be much lower than that of their predecessors, the barbiturates. Over the next 20 years the benzodiazepines became the most commonly prescribed of all drugs in the Western world. Their proprietary names, such as Librium, Valium, Mogadon, Ativan and Halcion, are now household words over much of the globe.
In many ways, the popularity of the benzodiazepine tranquillisers was a product of the permissive society. In their heyday, from the Swinging Sixties and into the Seventies, these drugs were increasingly expected by patients, and prescribed by doctors, for almost any stress, however minor. They were prescribed for a visit to the dentist, “exam nerves” and even for taking a driving test. By 1979, prescriptions for benzodiazepines in Britain had soared to reach more than 30 million (usually for a month's supply), for a population of about 55 million people. Malcolm Lader, professor of pharmacology at the Institute of Psychiatry in London, estimated that one in five of all women and one in ten of all men were taking benzodiazepines for some time each year, and many had taken them for years.
Only recently has the cost of all this drug-induced tranquillity become apparent. It is now clear that benzodiazepines are not without harmful effects, especially when taken over long periods. The tide has turned: there is now mounting public pressure for people taking tranquillisers to stop, and the media are full of the tragic stories of long-term users who have become dependent. Last year the Committee on Safety of Medicines warned the medical profession officially of their deleterious effects in the long term. Doctors have been advised to prescribe them with more circumspection, and prescriptions have declined by about 16 per cent in the past six years.
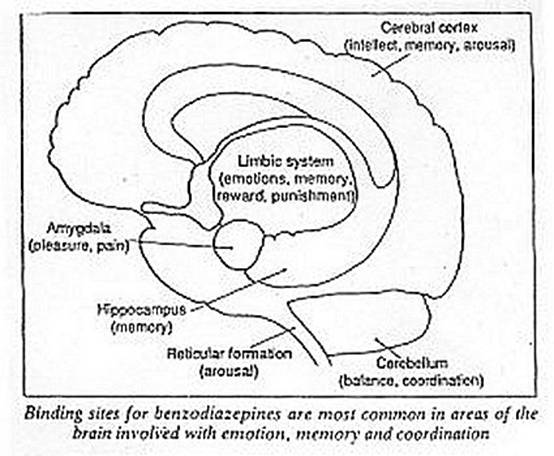
Nevertheless, the era of benzodiazepines has stimulated research into how they work, and this in turn has shed new light on the workings of the brain and on the neurophysiology of anxiety. The benzodiazepines exert several major effects. They work as hypnotics and sedatives, accounting for their efficacy as sleeping pills. They also relax muscles, and are anticonvulsants. All these actions result from the ability of the drugs to bind with specific sites in the brain. These sites, discovered independently in 1977 by H. Möhler and T. Okada at Hoffman-La Roche in Basel, Switzerland, and Richard Squires and Claus Braestrup at A/S Ferrosan, in Soeberg, Denmark, are unevenly distributed in the brain. They are particularly prevalent in areas concerned with emotional reactions, memory, thinking and the control of consciousness, as well as the regions involved in maintaining muscle tone and co-ordination. Binding sites for benzodiazepines appear in the brains of many vertebrates including fish, rats and apes as well as people.
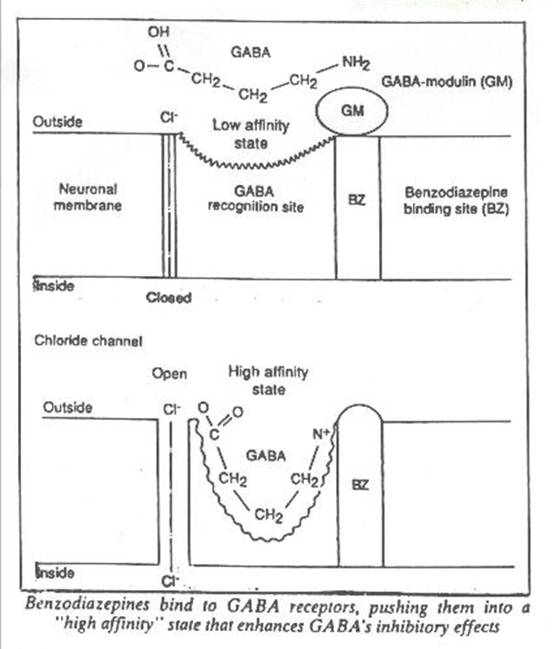
The specific binding sites for benzodiazepines are themselves part of a more complex unit comprising the receptor for a natural brain neurotransmitter, gamma-aminobutyric acid or GABA. GABA is a universal inhibitor, secreted by some nerve cells as they associate with other nerve cells to form a synapse. It inhibits directly the firing of neurons containing receptors for GABA, and indirectly the release of other brain transmitters including acetylcholine, noradrenaline, dopamine and serotonin.
The multimolecular unit that is the receptor includes binding sites for both GABA and benzodiazepines. Once GABA binds to it, the receptor changes shape. This in turn opens a chloride channel in the membrane of the neuron. Chloride ions enter the nerve cell, increasing the ionic gradient between the inside of the cell and its surroundings. This condition, hyperpolarisation, makes the nerve cell resistant to excitation; it is more difficult to get the cell to convey a nerve impulse, which is in essence a wave of depolarisation. So GABA secreted by one neuron can inhibit nerve cells further down the chain, producing so-called postsynaptic inhibition.
Not all the brain's inhibitory GABA synapses are active at once, however; otherwise, we would be permanently asleep. The GABA-receptor seems to exist in two states: a “high affinity” state that favours its reaction with GABA, and a “low affinity” state that discourages the binding of GABA. Work by Erminio Costa, A. Guidotti at the National Institute of Mental Health in Washington DC and others suggest that the receptor's affinity for GABA is normally controlled by a protein in the membrane, GABA-modulin. It appears that once a benzodiazepine occupies a binding site, it inactivates GABA-modulin, pushing the receptor into the high affinity state. This makes the receptor more keen to bind molecules of GABA, so enhancing GABA's ability to inhibit neurons.
Many of the actions of benzodiazepines probably result from such an enhancement of GABA's inhibitory powers. The drugs relax muscles because they inhibit some areas of the brain that control muscle tone. The anticonvulsant effects are due to the inhibition of the spread of electrical activity in the brain. The ability of benzodiazepines to induce sleep and tranquillity may also stem in part from the secondary damping of the release of other neurotransmitters, such as noradrenaline and serotonin, in excitatory pathways influencing arousal and emotional responses. Some researchers have even suggested that anxiety states may be the result of the underactivity of GABA in the brain. The neurotransmitters may fail in their suppressive activities because too many GABA-receptors remain in the low affinity state, or fail to show the normal protective increase in affinity for GABA under stress. Benzodiazepines would promptly reverse such a situation. It is interesting that barbiturates, other sleeping pills, alcohol and some antiepileptic drugs also enhance or mimic the activity of GABA in the brain. These drugs have similarities with benzodiazepines in that they induce sleep, reduce anxiety and convulsions as well as being liable to cause drug dependence.
The discovery of the binding sites of benzodiazepines in the brain led to the speculation that there might be an endogenous benzodiazepine, a natural tranquilliser equivalent to the endogenous opioids, the enkephalins or endorphins. We now know that these “natural painkillers” act on the same receptors as narcotic drugs such as morphine. But no convincing “endodiazepine” has yet appeared and Squires, one of the discoverers of the benzodiazepine receptor, believes that “there is no ‘endogenous ligand’ and no reason to assume that one exists”. Nevertheless, a number of endogenous substances, including some steroid hormones, can modulate GABA activity. Their significance is not yet clear.
Meanwhile, researchers have synthesised a series of compounds that bind specifically to benzodiazepine sites yet exert a spectrum of effects. Some act like benzodiazepines; others have the opposite effects and actually induce anxiety, insomnia or fits. Still others produce some but not all of the actions of benzodiazepines, antagonise one or more of their actions, or block some and promote others. All these substances probably modulate the activity of nerve cells that use GABA as a neurotransmitter, probably by altering the affinity state of the GABA receptors. But their range of activity suggests that different subtypes of receptors for GABA or benzodiazepines mediate different effects of the drugs. So it may be possible to develop benzodiazepine-like drugs that reduce only anxiety, or do nothing but induce sleep or stop convulsions.
A further subtlety in the actions of benzodiazepines is that they may also involve the opioid systems of the brain. Steven Cooper at the University of Birmingham found that he could block the ability of benzodiazepines to reduce anxiety in rats by giving them small doses of the drug naloxone, which blocks opioids. An interaction with the endogenous opioids provides a link with other drugs of dependence. Alcohol, for example, has analgesic effects like morphine, and some of its metabolites have narcotic properties. Nicotine, too, can release endogenous opioids. The receptors for both opioids and benzodiazepines are present in high numbers in the part of the brain known as the amygdala; located in the limbic system, it is concerned with the perception of pain and pleasure.
Quick relief for acute stress
Benzodiazepines are valuable drugs in the short term. They can save lives where people are suffering repeated or continuous convulsions caused by drugs or poisons. They are ideal as premedication before surgery and for minor operations, where they provide tranquil sedation as well as amnesia for the event. A few days' use in acute, severe anxiety may avert a crisis and allow time to arrange longer-term treatment. And occasional use may ensure a refreshing night's sleep at times of temporary disturbance.
The problems begin with chronic use: that is, regular use for more than a week or two. Harmful effects fall into two categories that may co-exist: the drugs may begin to have powerful but unwanted side-effects, and also to become less effective. The individual taking them may develop tolerance, dependence and the symptoms of withdrawal.
Unwanted effects can be pronounced. Many benzodiazepines are eliminated from the body only slowly and may accumulate causing drowsiness, a lack of co-ordination, impairment of memory and concentration, and confusion. These effects are most marked in the elderly, making them more likely to suffer falls and fractures. Benzodiazepines taken as sleeping pills often give rise to a “hangover”, impairing performance the following day. Benzodiazepines may contribute to traffic and industrial accidents and worsen the effects of other depressant drugs, including alcohol.
Taking benzodiazepines over the long term can cause both depression and “emotional anaesthesia”, an apathetic state in which people are unable to feel pleasure or pain. The drugs can aggravate depressive illness and provoke suicide. On the other hand, benzodiazepines sometimes produce apparently paradoxical stimulant effects. Patients may commit uncharacteristic anti-social acts such as shoplifting or sexual offences, or become aggressive with outbursts of rage and violence. Some researchers have suggested that chronic use may contribute to “baby-battering”, “wife-beating” or “grandma-bashing”. Clinicians have attributed these paradoxical effects to behaviour normally suppressed by social restraints, fear or anxiety, and they are most likely to happen in aggressive or anxious individuals. Alcohol is well-known to have a similar effect.
Another worry is benzodiazepines taken during pregnancy. The drugs readily enter the foetus and can depress vital functions in the new-born infant. Babies chronically exposed to benzodiazepines in the womb may develop withdrawal symptoms between two and three weeks after birth. The drugs also enter the breast milk if taken by a nursing mother.
Some researchers have suggested that the drugs can also cause brain damage. In one study, brain scans seemed to show that the brains of people who took benzodiazepines over the long term shrank. A later study did not confirm this finding, and this question remains open.
In the midst of such side effects is the phenomenon of tolerance. Tolerance of benzodiazepines develops rapidly, within days or weeks. When someone takes the drugs to reduce anxiety during the daytime, the sedative effects usually wear off within a week. A careful study by Malcolm Lader showed, however, that memory and reasoning abilities in regular users was below par, a fact that they did not recognise until they stopped the drugs. Tolerance to the anti-anxiety effects may develop more slowly. But Peter Tyrer, in a recent study of patients with anxiety disorders in Nottingham, found that benzodiazepines did little to reduce anxiety after people had been taking them for just four weeks. Similarly, benzodiazepines are generally unsatisfactory for the control of epilepsy over the long term because seizures tend to recur despite continuous use.
People become tolerant to benzodiazepines probably because their nerve cells respond by producing fewer receptors for GABA/benzodiazepines. This phenomenon, known as “down regulation”, means that the number of “high affinity” GABA receptors decreases in response to the enhancement of GABA caused by the drug. Such homeostatic responses, which tend to reinstate the status quo despite the continued presence of drug, happen with many of the drugs that people take regularly, including alcohol, opiates and even beta blockers, which are widely proscribed for heart disease. The adaptation of behaviour to overcome the actions of the drugs probably also contributes to tolerance.
Whatever the mechanism, the development of tolerance sets the scene for withdrawal effects. At this stage, the removal of benzodiazepines, or even a reduction in dosage, exposes the altered state of the brain, with fewer “higher affinity” receptors for GABA to act upon. Symptoms for which the drug was prescribed re-emerge and may often rebound to levels greater than originally present. A common example is rebound insomnia, which can happen after only a week of taking benzodiazepines as sleeping pills. During the rebound, the onset of sleep is delayed, and sleep when it comes is punctuated by vivid dreams, nightmares and frequent awakenings. (During use, benzodiazepines suppress dreaming and rapid-eye-movement, or REM, sleep.) Rebound insomnia can occur in people who are not initially insomniac but who have been prescribed benzodiazepines routinely during a stay in hospital.
Rebound insomnia is a mild example of the host of possible withdrawal symptoms from benzodiazepines. Sudden withdrawal from large doses may cause convulsions or precipitate an attack of highly disturbed behaviour. More commonly, the withdrawal syndrome consists of a whole panoply of anxiety symptoms: panic attacks, phobias, tremors, palpitations, feelings of unreality, perceptual distortions, hypersensitivity to light and sound, and many others. The syndrome appears in many patients even in controlled conditions, when neither patients nor doctors know that withdrawal is happening. The time at which symptoms begin corresponds to the rate at which the drugs are eliminated from the body, happening earlier with rapidly eliminated benzodiazepines and after a delay with slowly eliminated ones. Withdrawal symptoms may decline after a few weeks, but in some cases last for months.
So it is not surprising that some people become dependent on benzodiazepines. The initial relief of symptoms such as anxiety and insomnia motivates people to continue to take the drugs. When tolerance develops, and the drug becomes less effective, the onset of withdrawal symptoms make it difficult to stop. Such a cycle has been observed with many drugs of dependence, including nicotine, alcohol, barbiturates and heroin: drug-taking initially for relief or reward merges into continued drug-taking to avoid withdrawal symptoms. By this time, people need the drug for psychological and physical comfort and suffer if they try to stop. In the case of benzodiazepines, patients may get sucked into a spiral of continued drug use from which it is difficult to escape.
The effect of a drug depends not only on its properties but also on the characteristics of the individual. Phlegmatic people dislike the effects of benzodiazepines, finding them beneficial only in time of extra stress. Such people are unlikely to continue taking the drugs. In fact, fewer than 30 per cent of patients take the full course of a prescribed tranquilliser; fewer still return for a repeat prescription. But at least at first, benzodiazepines potently relieve feelings of stress in anxious people, and the greater the anxiety the better they work. Thus most people who take, and keep on taking, benzodiazepines are anxious. As Tyrer points out, such people are typically “timid worriers”, especially vulnerable to developing dependence and suffering withdrawal effects.
Such people take the drugs as a means of protection against stress. Ironically, the benzodiazepines make it more difficult to learn alternative strategies for coping with stress, such as behavioural treatment for agoraphobia. In addition, the drugs soon lose their ability to reduce anxiety, and may even make it worse, because of tolerance and withdrawal effects. Taken over a long time, benzodiazepines clearly create more problems than they solve.
Many of the 1 to 2 million people in Britain who have been taking tranquillisers for prolonged periods are now finding a way out. The answer in general seems to be a gradual tapering off the drug, combined with methods aimed at developing other ways of dealing with stress. A wide variety of methods—from cognitive and behavioural therapies, through yoga and relaxation, to simple information and common sense—can help. Better ways of coping with stress may involve modification, through learning, of the way the brain uses GABA. Doctors are increasingly aware of the need for slow, supervised withdrawal from the drugs, and support groups have sprung up throughout the country. The overall success rate of withdrawal from tranquillisers appears to be high, although no one has accurate figures. The process demands a high degree of self-motivation and patience.
The benzodiazepine saga provides a cautionary example of the dangers of the over-enthusiastic prescribing of psychotropic drugs. The market is now pregnant with a new generation of non-benzodiazepine drugs to reduce anxiety, reputedly innocent of dependence producing properties. Doctors and patients should use them with care, remembering that any drug that gives pleasure or allays suffering, whether prescribed like benzodiazepines or freely available like alcohol and caffeine, can cause dependence.
The users of benzodiazepines are not alone. There are few people who do not respond to their psychological needs with suitable doses of drugs. Who does not rely on a cup of tea or coffee to start the morning, and indeed repeat the dosage of caffeine throughout the day? Who does not occasionally assuage their cravings for sweet reward with a bar of chocolate? Millions wind down the day, or relax socially, with a benzodiazepine-like, GABA-enhancing alcoholic drink. The 30 per cent of the population who still smoke manipulate their dosage of nicotine to achieve either stimulant or calming effects. The main difference is that, unlike benzodiazepines, these drugs are not prescribed by doctors.
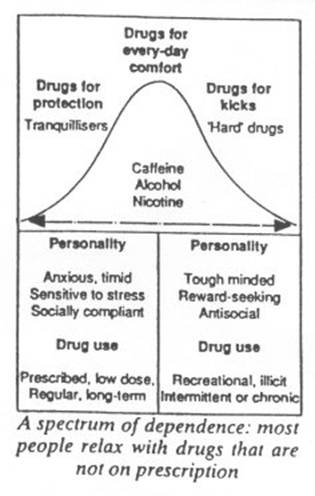
People who take tranquillisers in small prescribed doses as drugs of protection against stress constitute one extreme of a normal population. The other extreme consists of a minority who take large doses of non-prescribed drugs for “kicks”. These people tend to have a different personality make-up and a different social environment. They tend to be tough-minded, stimulus-seeking, and avid for immediate reward. Tranquillisers are occasionally abused by this group: some benzodiazepines taken in high doses can produce a “kick” and can also alleviate withdrawal symptoms from “hard drugs” such as heroin.
All drugs that are liable to abuse and can produce dependence affect the limbic system, the emotional centre of the brain. It is no coincidence that this area also houses the systems that signal reward and punishment. According to their original make-up and their environment, people tend to take particular drugs in the particular dosage that provides them with reward or protection. The same effects can, of course, be obtained by other means: music, art, physical exercise, a satisfying job, a loving relationship and a host of other human activities all provide their own rewards and tranquillity. The task for society, perhaps, is to provide opportunities for all to enjoy such non-pharmacological pleasures, reserving a few selected drugs (alcohol, caffeine?) to be savoured in moderation as icing on the cake. □






















