We are pleased to announce a formal benzo petition is now on the docket of the US Food and Drug Administration (FDA) and is open for public comment from everyone (not just US citizens). This document is not the older benzo petition some may have signed in the past. In this Citizen Petition we have asked the FDA to require more accurate prescribing guidelines and side effect information for all benzodiazepines.
Public comments WILL affect how the FDA rules on our requests--this is your chance to be heard. Please make the FDA aware of how benzodiazepine withdrawal has impacted your life. This is not a classic signature petition where you just sign your name. On this petition you can give a short statement of support to the FDA, or a more in-depth comment on the benzodiazepine issue; this is all done on-line and is an easy process.
SHORT INSTRUCTIONS ON SUBMITTING PETITION COMMENTS TO THE FDA
To comment on the petition click here Comment
(If you select 'individual consumer' from the pull down menu your comment will not be posted on the government website, only the FDA will see. When they ask for organization and third party just put N/A).
To read petition, click on the .pdf image here Read
DETAILED INSTRUCTIONS ON SUBMITTING PETITION COMMENTS TO THE FDA
| STEP ONE | READ the document | Click here - scroll down to 'Views' and click on the pdf image next to it |
| OR read the Summary of the Petition Requests | Click Here | |
| STEP TWO | READ a sample comment | Click Here |
| STEP THREE | READ instructions on submitting a comment | Click Here |
| OR see a sample form | Click Here | |
| STEP FOUR | WRITE a comment | Click here |
| STEP FIVE | EMAIL Us | benzo_action@yahoo.com |
Please encourage family, friends, and others who care about this issue to comment. Feel free to spread the word and this link--the more public comments the better.
The FDA will rule within six months on our requests, but we don’t know for sure when they will close the comment period. We deeply appreciate you making the effort to have your voice heard on this issue with a timely response. We hope with everyone's help there will soon be some much needed change in benzodiazepine drug labeling.
Emily, Shirley, and Anthea
PS: In order to track how many responses the FDA gets, we would appreciate you sending a quick email to: benzo_action@yahoo.com after voicing your support of this petition
SUMMARY OF PETITION REQUESTS: (Based on pg 3 of FDA petition)
In short, this petition is requesting all benzodiazepine drug labeling reveal that:
1. Benzodiazepines should not generally be prescribed for more than two to four weeks.
2. There is a potential to develop physical dependency after only one week of use.
3. There is a risk of developing protracted withdrawal with use of these medications.
4. Physical dependency and addiction are not the same, and require different treatments.
5. The newer benzodiazepines are much stronger than many doctors realize.
6. The proper way to taper can be much longer than many doctors realize.
Additionally, we have asked that:
1. A Black Box Warning is placed on this class of medication.
2. Leaflets to patients are given with each Rx filled, revealing the above information.
3. All medical providers are promptly notified of these changes.
SAMPLE COMMENT (Responses can be up to 2000 characters)
"To Whom It May Concern:
In 1995 I was prescribed clonazepam to treat undiagnosed dizziness and muscle pain. I took the medication until early 2006 precisely as prescribed by my doctors. At that point in time, my new pain management doctor (I had moved necessitating a change of doctors) insisted that I discontinue the drug practically cold turkey. I "tapered" from 3 mg to zero in slightly less than 1.5 weeks after having been on for more than 10 years. I thought that because a doctor had instructed me to do so, that meant it was safe to do so.
I was aware that clonazepam could cause dependence and anticipated a withdrawal of some sort, but nothing dangerous. Two weeks after my forced "taper," I seized and was reinstated onto the medication in the emergency room to prevent further seizures. I won't attempt to tell you what my life has been like since then. I will merely tell you what resulted. I lost my health, my career, my life savings, my status as a contributing member of society and I almost lost my marriage. I am STILL trying to taper off of this drug which has caused possibly permanent damage to my central nervous system.
The lack of proper labeling for this class of drugs (benzodiazepines) meant that, as a consumer, I wasn't able to make an informed decision on whether taking this drug, especially long term, was a wise idea. It also meant that the pain management doctor I had in early 2006 didn't know that taking me off the medication as quickly as he did was as dangerous as it turned out to be. The emergency room doctors and the other doctors I've seen since (with one notable exception) also seem unaware of the danger.
For this reason I urge the FDA to adopt the matters set forth in this petition; to more clearly warn both patients and doctors about the possible consequences of long term benzodiazepine usage and to adopt a black box warning for this class of medication.
Sincerely,
Jane Doe
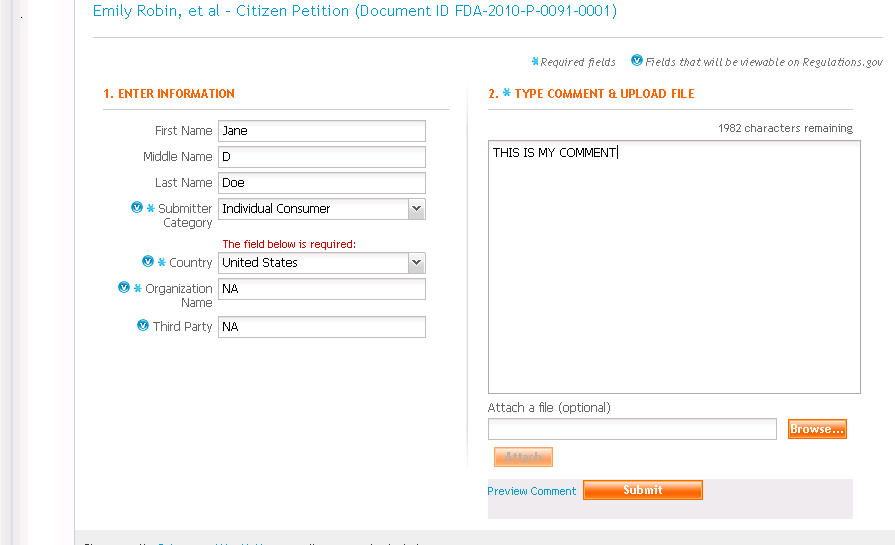
INSTRUCTIONS ON SUBMITTING COMMENTS TO THE FDA (for benzo petition):
1. To submit your comments, keep the following in mind:
After filling in your name, in the ‘Submitter Category’ from the pop down box choose either:
‘Individual Consumer’ if you want your comments to remain private ‘Consumer Group’ if you want your comments viewable on that site .
In the boxes ‘Organization Name’ and ‘Third Party’ type in ‘NA’
2. You can add your comments three ways:
- Type directly in the box (maximum 2000 characters)
- Type as a separate document then cut and paste into the box (max 2000
characters)
- Type it as a separate document and attach it (no limit on characters); in the
message box say ‘see attached document.’
TIP: If you plan on taking some time writing your comments, you will want to either cut and paste them in the box or attach as a separate document, as some have said the system times out on them, in which case you have to start over.
3. Click the ‘Submit’ box:
If it was received, you will see ‘Success!’ and get a tracking number If
you don’t get that, try again, or click on the ‘Help Desk’ button
It really isn’t complicated—it’s actually very easy!
Just be sure your comments go through by getting the tracking number, or asking for help if you have trouble.